 Synthesized steroid found naturally in the spiny dogfish, a species of shark, prevents the accumulation of a protein that is implicated in neurodegenerative diseases according to a study published in animal models. The accumulation of this protein, alpha-synucleinis the signature of Parkinson’s disease and dementia with Lewey bodies. This could be a potential new component for therapeutic research.
Synthesized steroid found naturally in the spiny dogfish, a species of shark, prevents the accumulation of a protein that is implicated in neurodegenerative diseases according to a study published in animal models. The accumulation of this protein, alpha-synucleinis the signature of Parkinson’s disease and dementia with Lewey bodies. This could be a potential new component for therapeutic research.
The work, published in the journal Proceedings of the National Academy of Sciences, also demonstrated that the synthesized steroid, known as
squalamine
reduced the toxicity of existing alpha-synuclein clusters.
The results of the pre-clinical study show that squalamine prevents and eliminates the accumulation of alpha-synuclein in neurons by loosening the protein from the inner lining of nerve cells, which is where it clings and forms toxic clumps. The animal model used in this study, C. elegans, is a nematode worm that is genetically modified to produce human alpha-synuclein in its muscles. As these worms age, the accumulation of alpha-synuclein in their muscles causes cell damage and paralysis.
You can literally see that squalamine, which is given orally to the worms, prevents the accumulation of alpha-synuclein and it also prevented muscle paralysis in the worms, according to Michael Zasloff, senior co-author of the study and professor of surgery and pediatrics at Georgetown University School of Medicine.

Zasloff, an expert in innate immune systems, has been studying squalamine for 20 years. He discovered it in spiny dogfish in 1993 and synthesized it in 1995 and the process does not involve any natural shark tissue. His research, as well as work by other researchers, has established antiviral and anti-cancer properties of the compound. This is the first study to suggest neurological benefits in in vivo models of Parkinson’s disease.
In Parkinson’s disease, alpha-synuclein, a normal protein found in the nervous system, forms toxic clusters that damage and destroy the neurons on which they form. There is a lot of research to discover components that prevent the formation of these masses. In this study, the researchers demonstrated in a series of in vitro experiments that squalamine, a positively charged molecule with a high affinity for negatively charged members, could literally expel alpha-synuclein accumulation from negatively charged membranes, thus preventing the formation of toxic clusters.
According to Dr. Zasloff: The initial focus was on Parkinson’s disease due to a clear relationship between Squalamine function and the pathophysiology of Parkinson’s disease. We believe that there are other neurological conditions that could be treated with Squalamine, but our clinical trials will focus on Parkison and the non-motor symptoms of this disease.
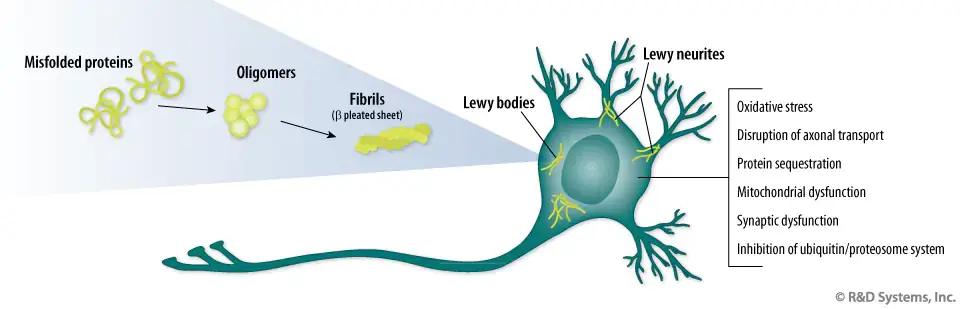
The team also demonstrated that squalamine could protect healthy neuronal cells from damage by already-formed alpha-synuclein masses by preventing them from adhering to the outer limb of neuronal cells. The researchers then extended their studies to living systems, C. elegans, which are common animal models in Parkinson’s disease. Zasloff concludes: Oral administration of squalamine prevented the formation of toxic alpha-synuclein clusters in this complex animal and saved it from paralysis. This experiment shows that the mechanism discovered in vitro achieved the predicted result in an animal.
The study of squalamine for its properties has been the subject of research for several years. In 1998, research suggested beneficial effects of squalamine on tumor development in in vivo animal models. There is also a phase 1 clinical trial that measured the toxicity dose of squalamine with the conclusion that the compound could be used in late stage lung or ovarian cancer while respecting the toxicity doses in humans. It should be noted that the recommendation of the researchers in this 2001 study concerns only phase 2 clinical trials.